Development and validation of a work quality questionnaire in medical biochemistry labs
DOI:
https://doi.org/10.17532/jhsci.2025.2887Keywords:
Laboratory staff, quality control, questionnaireAbstract
Introduction: Medical biochemical laboratory professionals play a critical role in diagnostics, research, and patient care, performing complex tasks that require extensive knowledge, professional attitudes, and adherence to best practices. Understanding their knowledge, attitudes, and practices (KAP) is essential for improving laboratory performance, ensuring quality, and enhancing patient outcomes. Despite the importance of quality control systems and international standards, the existing literature reveals a lack of validated instruments to assess KAP among laboratory professionals. This study aimed to develop and validate a comprehensive questionnaire targeting key domains of laboratory practice, with the goal of identifying operational gaps and guiding future interventions.
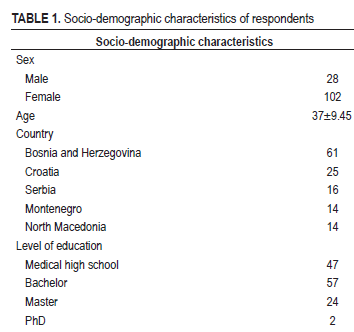
Methods: The questionnaire was developed through a four-phase process: Literature review, item construction, questionnaire distribution, and validation. Psychometric evaluation included internal consistency testing and factor analysis to ensure reliability and validity.
Results: The final instrument, titled KAP of Laboratory Professionals on Standards and Work Quality Systems, comprised 73 items across six domains. The overall Cronbach’s alpha was 0.673, indicating moderate but acceptable internal consistency. The questionnaire effectively identifies gaps in KAP related to quality control in medical-biochemical laboratories. Its results can support laboratory managers in recognizing areas for improvement, ultimately enhancing service quality and patient outcomes.
Conclusion: This descriptive and analytical study presents a validated and reliable tool for assessing KAP regarding standards and quality control systems in medical-biochemical laboratories. Its application can guide targeted interventions to address deficiencies and strengthen practices in laboratory medicine.
Downloads
Downloads
Published
License
Copyright (c) 2025 Aleksandra Pašić, Arzija Pašalić, Amra Mačak Hadžiomerović, Sabina Šegalo

This work is licensed under a Creative Commons Attribution 4.0 International License.










